
Chung Research Group Sustainable and Biomedical Polymers

Functional Bottlebrush Adhesives and Polymers
Adhesives are nonmetallic polymeric materials that join two independent surfaces through chemical and/or physical bonding. Despite their critical role in advanced technologies, most existing adhesive products remain limited: they are typically single-purpose, single-material, and singular in function. The Chung group develops novel synthetic strategies and investigates structure-property relationships of multi-functional adhesives designed to surpass the conventional role of adhesives. Our research spans bottlebrush adhesives, biomedical adhesives, stimuli-responsive, and electrically conductive adhesives (ECAs). Drawing inspiration from marine organisms, we integrate 3,4-dihydroxy-L-phenylalanine (DOPA) and seashell nacre multiphasic structure into synthetic polymers to overcome the limitations of current adhesive technologies.
One work of ours that highlights our focus on bottlebrush polymer adhesives demonstrates a strategy to engineer tough, salt-tunable adhesives by exploiting weak electrostatic interactions within polyzwitterions (PZIs). Two topologies—bottlebrush (BB-PSBMA) and linear (L-PSBMA) were synthesized from sulfobetaine methacrylate and exhibited comparable adhesion strengths (~0.4 MPa). However, their response to NaCl revealed distinct toughness behaviors: BB-PSBMA transitioned from strong-brittle to strong-ductile with a 77% increase in work of debonding at 70 mM NaCl, while L-PSBMA shifted primarily from brittle to ductile. The enhanced performance of BB-PSBMA was attributed to a synergy between viscosity-governed adhesion–cohesion balance and sacrificial electrostatic associations that dissipate mechanical energy. These findings highlighted how precise control over polymer architecture and salt concentration can create multifunctional adhesives with tunable strength and toughness.
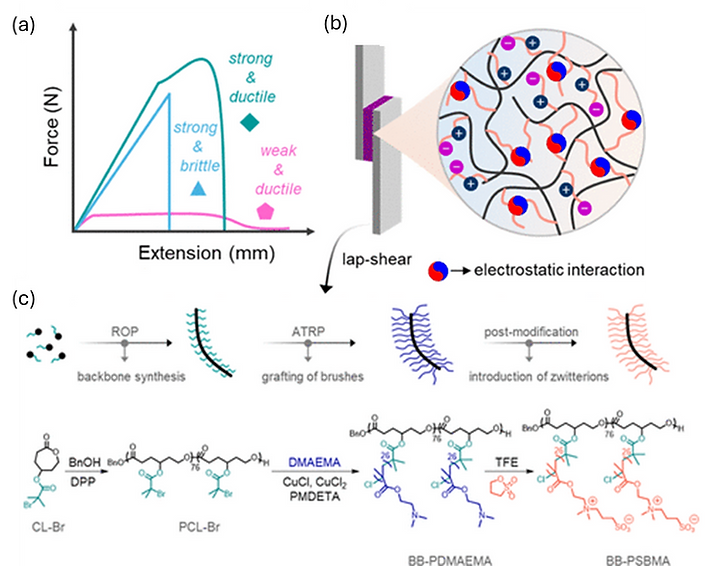
Figure 1. (a) Demonstration of the effect of architecture and salt concentration on the tunability of this adhesive’s properties. (b) Effect of salt to disrupt the electrostatic interactions governing the adhesion properties. (c) Synthesis scheme of bottlebrush adhesive BB-PSBMA.
Biomedical adhesives are a rapidly growing field because of the potential applications in tissue adhesion, wound closure, hemostatic agents, wound dressing, and drug delivery. However, it is very hard to find a single chemical structure that possesses the multifunctionality required for biomedical adhesives (e.g., strong internal organ tissue adhesion and controlled drug delivery). Our group has developed several classes of bioinspired biomedical adhesives that harness mussel-inspired catechols and complementary chemistries to achieve strong, biocompatible, and tunable adhesion. A fast-acting adhesive based on poly(AMPS-co-MDOPA) demonstrated rapid skin bonding, excellent cytocompatibility, and effective drug delivery, highlighting its potential for topical therapeutic applications (Figure 2a). A glucose-based adhesive incorporated catechol groups and clickable azide moieties, enabling controllable adhesion strength through SPAAC crosslinking while maintaining non-toxic performance (Figure 2b). Complementing these designs, a lightly crosslinked zwitterionic adhesive combining SBMA, MDOPA, and PEGDMA provided robust wet adhesion for soft tissues, successfully sealing intestinal anastomoses ex vivo without cytotoxicity (Figure 2c). Together, these advances demonstrate a versatile toolkit of synthetic strategies for creating next-generation biomedical adhesives that balance strength, flexibility, and biocompatibility across diverse clinical needs.
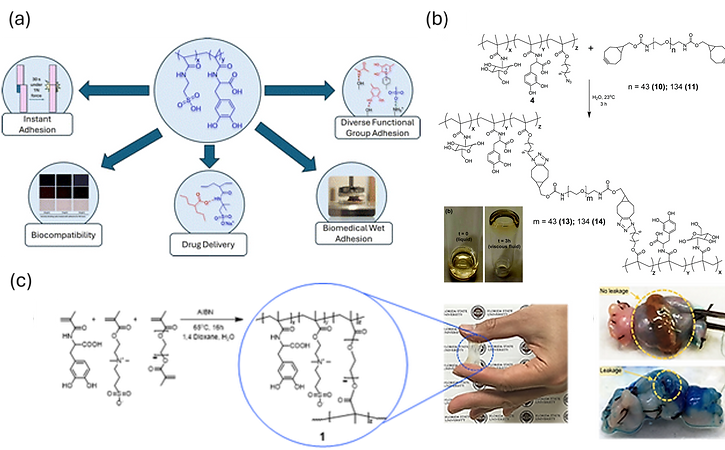
Figure 2. (a) Biomedical adhesive poly(AMPS-co-MDOPA) diverse application branches due to novel copolymer structure of polyelectrolyte AMPS and mussel-inspired MDOPA. (b) SPAAC inspired azide alkyne crosslinking for strong biomedical adhesion. (c) Biomedical adhesive terpolymer utilizing zwitterionic moiety for kidney anastomosis prevention
The conventional ECA includes heterogeneous metallic and carbon-based additives in an organic adhesive polymer matrix. However, these heterogeneous ECAs with additives have serious issues with their aggregation, heavy weight, large filler content, rapid aging, toxicity, low adhesion/conductivity, poor mechanical properties, and high price. The new ECA solved these issues by employing a new molecular structure of flexible anionic copolymer complex to polythiophene (Figure 3). The following 5 areas will receive significant and direct benefit from the novel ECAs: battery binders, photovoltaics, electronic sensors, displays, and coating technologies.

Figure 3. (a) Chemical structure of new ECA, (b) High conductivity; LED light on by connection with the ECA. The ECA was flexible and it operates well after bending. (c) Strong adhesion; 12mg of adhesive holds 15 lbs bowling ball.