
Chung Research Group Sustainable and Biomedical Polymers

Lignin-based Sustainable Polymers
We developed an innovative and cost-effective ionic polymer for CO2 capture and utilization for the first time, using abundant and nonfood-based biomass lignin. The modified lignin-based ionic polymer is employed for CO2 capture from both direct air and concentrated CO2 sources at room temperature and atmospheric pressure. The captured CO2 is precisely quantified by using inverse-gated proton decoupled 13C NMR with an internal standard (trioxane). Remarkably, the captured-CO2 amounts of ionic lignin polymer are 1.06 mmol g−1 (47 mg g−1) from concentrated-CO2 source and 0.60 mmol g−1 (26 mg g−1) from direct-air. The captured-CO2 in ionic lignin polymer is released in controlled manner and utilized in the synthesis of cyclic carbonate, showcasing the productive application of the captured carbon. Moreover, the fully controlled recovering of ionic lignin polymer achieves via repeated CO2 release ↔ CO2 capture (Figure 1).
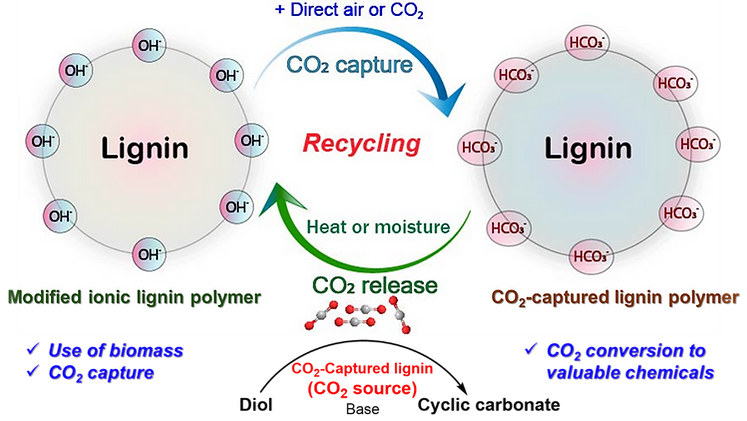
Figure 1. Repeatedly usable process of the modified ionic lignin polymer via continuous CO2 release ↔ CO2 capture cycles.
We established a conversion method of chaining up greenhouse gas CO2 with biomass lignin to develop new sustainable, recyclable polymers from abundant and non-food based renewable resources. A cyclic carbonate monomer has synthesized using a cost-effective, non-phosgene-based, and greener approach under atmospheric pressure and room temperature. The fully programable ring-opening polymerization is accomplished by varying the catalyst, catalyst loading and reaction time. Using catalysts, we achieved high-molecular-weight polycarbonates (Mn up to 155 kDa) with tunable thermal properties (Tg). These polymers are chemically recyclable, depolymerizing back to monomers under mild conditions, thus enabling a closed-loop recycling (Figure 2).

Figure 2. Graphical representation of closed-loop chemical recycling of CO2 and lignin-based polymer ↔ monomer.
In parallel, we explored the synthesis of polyhydroxyurethanes (PHUs) from lignin-based monomer and bio-based reagents for the first time without any catalyst (Figure 3). This is termed as non-isocyanate polyurethanes (NIPUs), which eliminate toxic reagents and offer excellent mechanical and thermal performance. Moreover, significant achievement includes the successful introduction of high and controllable flexible poly(dimethylsiloxane) unit to lignin-based polymers which are naturally highly rigid. Our approach demonstrates the feasibility of integrating CO2 integration into polymer synthesis, advancing the development of safe, scalable, and sustainable alternatives to conventional polyurethanes.

Figure 3. Reaction scheme for the CO2- and lignin-based polyhydroxyurethanes.
Our work on lignin-derived polyesters focuses on transforming lignin into functional monomers for thermoplastic applications (Figure 4). We developed a one-pot modification method using succinic acid and acetic acid to introduce carboxylic functionality while controlling crosslinking density. Acetic acid acts as a capping agent, selectively deactivating hydroxyl groups and enabling precise control over polymer architecture. The modified lignin was grafted with poly(ethylene glycol) (PEG) via carbodiimide-mediated esterification, yielding lignin-graft-PEG copolymers with tunable thermal properties (Tg: –27.5 °C to 2.6 °C) and improved solubility.
Further, we synthesized lignin-graft-castor oil copolymers using pristine castor oil and succinic acid-modified lignin (Figure 4). This 100% biomass-based polyester exhibits thermoplastic behavior with adjustable mechanical properties (Young’s modulus up to 228 MPa) and thermal transitions (Tm: 57–71 °C; Tg: 39–60 °C). The degree of crosslinking and grafting density directly influence crystallinity, density, and surface hydrophilicity, as confirmed by PXRD, DSC, and contact angle measurements. These materials demonstrate the potential of lignin as a versatile building block for biodegradable and recyclable polyesters, offering a scalable route to replace petroleum-based plastics.
