
Chung Research Group Sustainable and Biomedical Polymers

Fundamental Polymer Science
The Chung group’s research is centered on the advance of fundamental polymer science through the meticulous design, synthesis, and functionalization of precision macromolecules with tailored properties for biomedical and materials applications. The development of innovative polymer architectures, including bottlebrush polymers and functional macromonomers, has enabled the exploration of site-specific bioconjugation, tunable thermoresponsive behavior, and post-polymerization modifications for the control of bulk material properties.
For instance, 1) we established a site-specific route to protein–polymer conjugates by building azido-functionalized zwitterionic bottlebrushes via RAFT/ATRP, installing trans-cyclooctene by SPAAC, and ligating to tetrazine-labeled urate oxidase through rapid IEDDA click. The resulting UOX–bottlebrush conjugates demonstrated the ability to preserve enzymatic activity while significantly increasing proteolytic resistance and reducing antibody binding, illustrating how architecture and bioorthogonal chemistry can be employed to deliver protein therapeutics that exhibit retained enzymatic activity, enhanced proteolytic resistance, and reduced immunogenicity. In parallel, 2) we investigated the design of polymers with precisely defined stimuli-responsiveness and supramolecular interactions. Our research on thermoresponsive sulfoxide-containing methacrylate homopolymers has revealed two distinct mechanisms (LCST behavior driven by sulfoxide–water H-bonding & UCST behavior governed solely by sulfoxide–sulfoxide dipole–dipole complexes). The responsive system can be tuned near physiological temperature by pendant ester hydrophobicity and dipole disruptors. In addition, 3) we synthesized PEG-based bottlebrush polymers via graft-through ROMP featuring internal ketones. These ketones facilitate high-yield oxime ligation and subsequent metal-free click reactions, rapidly forming soft hydrogels including catechol-derived adhesive brushes.
These studies illustrate the integration of modular macromonomers, controlled architectures, and orthogonal chemical strategies to achieve programmable functional polymer systems, thereby facilitating avenues for the development of next-generation drug delivery vehicles, smart materials, and adaptive polymer networks.
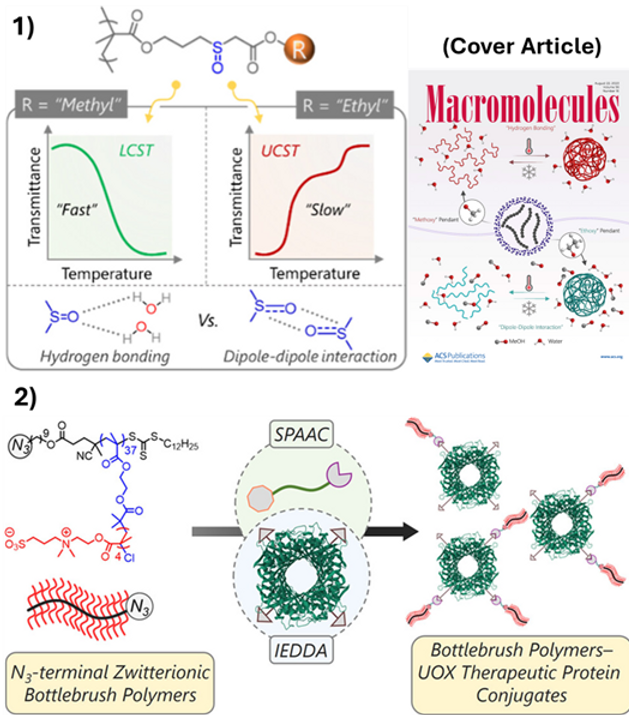
Figure 8. Water soluble Ru benzylidene catalyst’s chemical structure and catalyst recycle/removal principle via host-guest interaction and extraction.
The Chung group’s catalyst research focuses on organometallic reactions in neat water, the development of recyclable and removable catalysts that expand the frontiers of aqueous and environmentally friendly reactions.
1) The development of PEG-tethered Ru–NHC benzylidene catalysts has enabled the facilitation of controlled ATRP in neat water of commonly used acrylates (e.g., acrylamide, SBMA, PEGMA). These water-soluble complexes exhibit hallmark ATRP behavior, including first-order kinetics, linear growth of molecular weight with conversion, and low dispersity at very low catalyst loadings. This method provides a cost-effective and sustainable route to controlled radical polymerizations directly in aqueous systems. Moreover, these processes have been observed in organic media, such as acetonitrile. This platform is an early example of Ru-benzylidene–catalyzed ATRP in pure water, thereby advancing environmentally benign, cost-efficient routes to functional polymers with clear relevance to biomedical and high-performance applications where water compatibility and process sustainability are critical.
In parallel, 2) we have investigated the effect of ethylene-glycol–oligomer lengths on Ru–NHC olefin-metathesis catalysts to modulate dual solubility, aqueous reactivity, and—importantly—catalyst removal. While oligomer length exhibited minimal influence on metathesis kinetics in organic media, an increase in polarity resulting from longer glycol chains led to enhanced activity in water and a substantial improvement in aqueous extraction as a catalyst removal method. This simple yet effective solubility-driven approach achieved catalyst removal efficiencies that were comparable to or superior to those of conventional host–guest methods.
These studies indicated the potential for water-compatible, readily recoverable Ru catalysts that not only facilitate controlled polymer synthesis in benign conditions but also address the long-standing challenge of efficient catalyst recovery. This advancement contributes to the sustainable practice from synthesis through purification.
